After 48 years of service, MECO Vice President Robert "Bob" Gray is retiring.

Downloads
Recently retired Pfizer Executive Vice President Anthony Maddaluna sat down with Pharmaceutical Engineering to discuss his 42 years in the industry, his passion for excellence, and the complexities of supply chain organizational structure.
A Matter of Well-Being
Cover: Recently retired Pfizer Executive Vice President Anthony Maddaluna sat down with Pharmaceutical Engineering to discuss his 42 years in the industry, his passion for excellence, and the complexities of supply chain organizational structure.
Continuous Manufacturing
Special Report: PE contributors discus findings, outcomes, and the regulatory Q&A session from the ISPE Continuous Manufacturing Conference held 20–21 April 2016 in Baltimore, Maryland.
Corrosion Investigation of Pharmaceutical Clean Steam Systems
Facilities and Equipment: Corrosion byproducts encountered in clean and pure steam systems are present to some degree in every system, but many lack routine inspection and maintenance that could control corrosion and particulate migration from oxide deposit exfoliation.
In This Issue
Understanding the fundamental assumption of independence (and the violation thereof) enables an appropriate response to control chart trend rule violations and challenges us to think differently about special cause variation in control charts.
Much has been published on advantages of predictive maintenance, yet many do not realize that it is a tool to help achieve their facility's goals and objectives. If you need to convince management of the need for PredM, this article contains information that you can use as an "elevator speech."
This article presents current research on the problem of rouge in clean steam generators and their distribution systems, as well as possible deleterious effects on capital equipment and final drug products.
This paper discusses the findings and outcome of the ISPE Continuous Manufacturing Conference held 20–21 April 2016 in Baltimore, Maryland. While the ideas captured below reflect presentations and discussions both during the main conference and in breakout sessions, they are not necessarily the views of the authors or their organizations.
Hi David, I've had several interviews, but have yet to receive an offer. What could I be doing wrong?
Imagination is more important than knowledge. Knowledge is limited. Imagination encircles the world.
—Albert Einstein
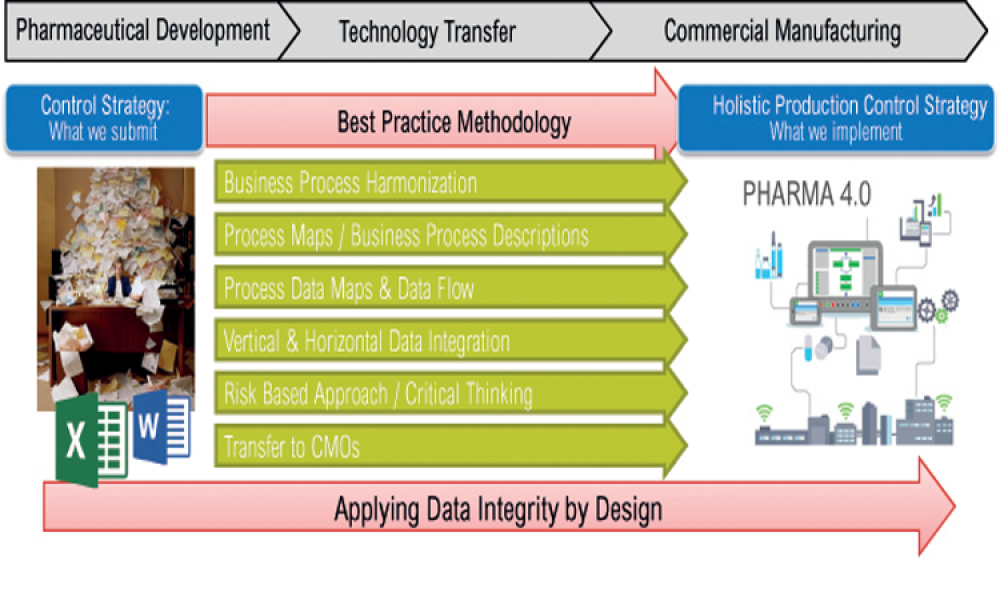
This article presents the work of the newly formed ISPE Holistic Production Control Strategy Working Group, which has identified and summarized the need for a redefined control strategy implementation methodology.