Just around the corner on 11-12 December, join us for a wealth of knowledge sharing, uniting industry experts to engage in discussions that unveil their insights and experiences through the 2023 ISPE Pharma 4.0™ and Annex 1 Conference.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
On Wednesday, 18 October 2023, Dr. Celia Lourenco, Associate Assistant Deputy Minister of Health Canada's Health Products and Food branch, presented a keynote address titled “Advanced Manufacturing and Other Trends Supporting Access to Medicines for Patients” at the
In the intricate world of pharmaceuticals, where precision and safety are paramount, the marriage of innovation and sustainability is not just an aspiration—it's a necessity. Picture a scenario where the pursuit of product purity meets the responsibility to our planet. Welcome to the realm of "Eco-friendly Pharma: Sterilization and Sustainable Practices." As we embark on this exploration,...
There are opportunities abound to transform inefficient production processes from the supply chain through the entire manufacturing process using existing and new digital technologies to significantly enhance overall operational efficiencies. Discover the cutting-edge advancements in these essential business areas at the
The 2023 ISPE Annual Meeting and Expo was a memorable gathering. With a blend of approximately 2,500 in-person and virtual attendees, the event was a melting pot of pharmaceutical professionals....
As the 2024 ISPE Aseptic Conference Chair, Christa Myers, Sr. Fellow - Aseptic and Sterile Products, Vertical Market Leader at CRB gets asked many times: what’s next? What are the new trends? Can Aseptic manufacturing change be easier, more cost-effective, and manage risks better? What are the inspectors looking for? Why can this not be more clear? Let’s find those answers together.
In recent years pharmaceutical manufacturers have responded to increased demands for speed to market, rapid drug development, and increased manufacturing capacities with innovative approaches to adapt to quickly shifting global needs. The design and construction of pharmaceutical facilities has evolved as companies identify ways to prepare new and existing facilities for future needs and...
During the 2023 ISPE Membership Meeting and Awards Lunch, Michael Rutherford, the outgoing Chair of the 2022-2023 ISPE International Board of Directors, officially handed over the gavel to Scott Billman. As the new Chair for the 2023-2024 term, Scott Billman, Vice President, Engineering, Pharmaceutical Services, Thermo Fisher Scientific, will lead the board. Rutherford expressed his gratitude...
Over 2,600 attendees from across 30 countries and a remarkable 200 exhibitors convened at the 2023 ISPE Annual Meeting & Expo in Las Vegas, NV, USA.
Close to 500 people celebrated the 2023 Facility of the Year Award (FOYA) Winners at a reception and banquet Sunday night, 15 October, during the 2023 ISPE Annual Meeting & Expo at Mandalay Bay in Las Vegas, Nevada, USA. Genentech was announced as the 2023 FOYA Overall Winner.
A fireside chat featuring Robert M. Califf, MD, FDA’s 25th Commissioner of Food and Drugs, and Thomas B. Hartman, President and CEO of ISPE kicked off the 2023 ISPE Annual Meeting & Expo. The following is a brief summary of the session. Look for complete coverage of the interview in an upcoming issue of Pharmaceutical Engineering® magazine.
Through the ISPE Foundation Diversity Internship Program, junior undergraduate and graduate-level students are afforded an opportunity to immerse themselves in a summer of groundbreaking engineering projects at some of the most cutting-edge facilities. This experience not only fosters the development of invaluable technical skills, but also serves as a first chance for students to establish a...
As the 2023 ISPE Annual Meeting & Expo in Las Vegas, NV, USA draws near, anticipation builds among the 110 students and Emerging Leaders from around the world who will be in attendance. These exceptional...
The 2023 Facility of the Year Awards (FOYA) Honorable Mention recognizes projects that did not win a specific category but were clearly successful projects that overcame significant challenges in planning, execution, and delivery.
The 2023 Facility of the Year Award (FOYA) Pharma 4.0™ category recognizes companies that embody the Pharma 4.0™ concept. This includes not only implementing one or more technological innovations, but also demonstrating the ability to change their culture, processes, and people orienting them towards a 4.0 future....
The countdown has begun, and the anticipation is evident as we gear up for the highly anticipated 2023 ISPE Annual Meeting & Expo. As we look back at the successful conferences over the past years, this event promises to be nothing short of extraordinary, especially for students and Emerging Leaders.
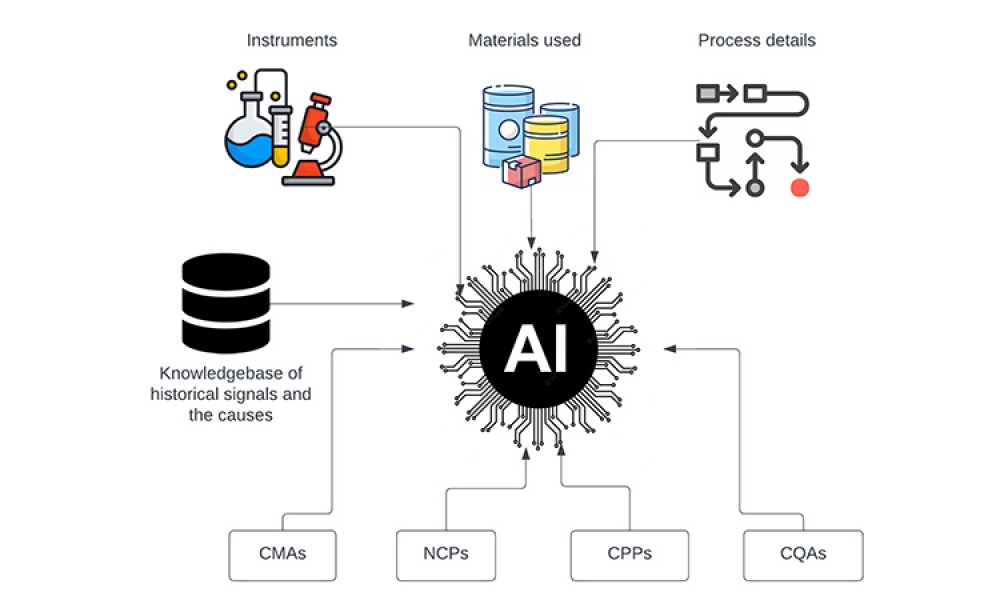
Continued Process Verification (CPV) was introduced roughly 12 years ago by the FDA as a third stage in the guidance on the process validation life cycle. In addition to being a mandatory requirement for the pharmaceutical industry, CPV can also provide valuable information to improve the quality and consistency of products. There may be limited data available when a process is designed and...