Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing Part 5

This article presents a consensus standardized extractables testing protocol for single-use systems in biomanufacturing
This article was originally published in the November – December 2014 issue of Pharmaceutical Engineering® magazine. Click here for access to Part1, Part 2, Part 3, and Part 4,.
Extractables Test Report
This standardized extractables testing protocol provides suppliers with a set of procedures agreed upon as representative of a comprehensive range of conditions by a broad group of companies. Suppliers can then prepare standardized extractables test reports for SUS components, including, but not limited to, bags and films, tubing, tubing connectors and disconnectors, aseptic connectors and disconnectors, sterilizing-grade and process filters, tangential-flow filter cassettes, sensors, valves, chromatography columns, molded parts of mixers, and filling needles. The extractables test report provides comprehensive information on the SUS component tested, including materials of construction, details of the testing setup, testing conditions and analytical methods applied, and identity and quantity of extracted compounds. The extractables test report should include the following information for each extractables study:
1. Summary Extractables Statement
The summary extractables statement for SUS components tested should consist of:
- Summary of the materials of construction
- Testing setup
- Extraction conditions applied
- Analytical methods applied
- Identity and quantity of extracted compounds (analytical results)
2. Details of pretreatment methods
- Gamma irradiation description includes the minimum and maximum dose allowed for the component during the manufacturing process and the actual dose used to gamma irradiate the component for testing.
- Autoclave description should include time (total and exposure) and temperature of each autoclave cycle. If multiple cycles are performed, the number of cycles also should be specified.
- Preflush description includes flush fluid used, temperature, time, and flush volume. Preflush typically applies to components such as tangential flow filtration cassettes.
3. Time intervals between manufacture, irradiation, and testing for gamma-irradiated bag films
The time interval between when a bag film is manufactured and when gamma irradiation is applied should be recorded. The additives in polymers used to make bag films can oxidize over time, and the oxidized additives can generate different extractables compared to virgin additives. The time interval between gamma irradiation and extraction also should be reported.
4. Thickness of the bag films and tubing
Multiple thicknesses of bag films are often available, e.g., 0.05 mm, 0.15 mm, and 0.5 mm. The thickness of the bag film or tubing should be reported.
5. Composition of fluid-contacting surface materials
Materials comprising the surfaces that contact test solvents during testing, e.g., the inner surface of tubing or connectors, the interior of bioprocess bags as well materials of construction of other layers, or the fluid-contacting components of filters, should be specified.
6. Traceability of components
The part numbers and lot numbers of test articles should be reported. These numbers should be traceable back to the lot numbers of resins used in manufacturing of the tested component.
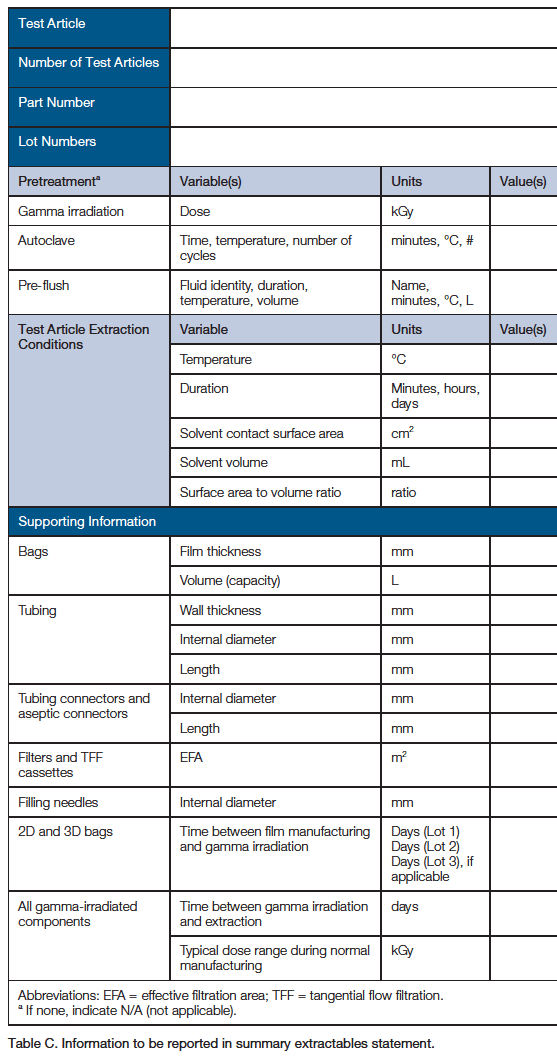
For each extractables study, the following information in Table C is recorded and included in each summary extractables statement. Results of extract analysis (compound identities and amounts) are recorded separately.
Next Steps
The companies involved in the BPOG Extractables Work Group encourage the adoption by all SUS suppliers of the recommendations made in this article. Not only will adoption enable results from extractables testing on SUS components to be compared and used by SUS integrators and end users, but also will simplify the approach of SUS suppliers to serve their markets. Such standardization will provide a set of common expectations for SUS component performance that SUS end users, SUS suppliers, and regulators can reference as the current good extractables testing practice.
This standardized extractables testing protocol also will be made available to standard-setting organizations, such as ASTM and USP for consideration in developing a consensus standard. We expect that once a consensus standard has been agreed upon that a transition plan will be created with reasonable timeframes permitting suppliers to bridge any existing gaps between the new standard and their existing extractables testing and documentation procedures.
Appendix: Recommended Analytical Techniques for Extractables Identification and Quantification
Outlined below are the recommended approaches for the four major analytical techniques applied to the identification and quantification of extractables from SUS components.
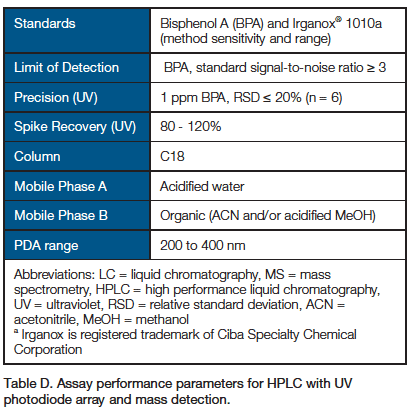
1. Detection of Extracts by LC-UV-MS: HPLC with UV Photodiode Array Detection and Mass Spectrometry
Notes:
- Other chromatographic instrumentation, such as ultra-high-performance liquid chromatography (UHPLC) and conditions may be used to meet assay performance parameters.
- Limit of quantitation (LOQ) should be reported.
- Standards listed in the table are to demonstrate method sensitivity and chromatographic range. Additional known extractable compounds should be prepared as standards injected for each unique material.
- An injection of standard should occur at least once for every 10 sample injections.
- Spike is 1 ppm BPA in water and in 50% water/50% ethanol.
- Control sample injections should be run to subtract matrix-associated peaks from consideration.
- Report levels of peaks from samples that are also observed in controls ≥ 50% higher than in controls.
- Mass spectrometric detection is both ± electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI).
- Mass spectrometric detection scan range is 100 to 2,000 m/z.
- In cases where quantitation is not possible, semiquantitative values may be reported by reference to responses of suitable standards.
- For semiquantitative analysis, results for peaks with a signal-to-noise ratio > 10 or peaks above area of lowest standard injection should be reported.
2. Detection of Extracts by GC-MS: Direct Injection Gas Chromatography with Mass Spectrometry
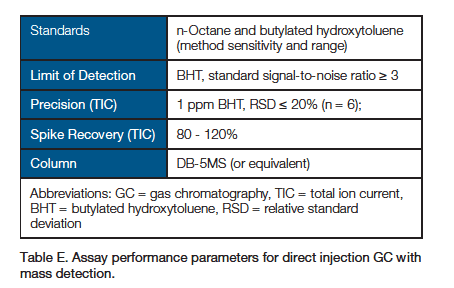
- Other chromatographic instrumentation and conditions may be used to meet assay performance parameters.
- Chromatographic data should be presented using the total ion current (TIC).
- Limit of quantitation (LOQ) should be reported.
- Standards listed in the table are to demonstrate method sensitivity and chromatographic range. Additional known extractable compounds should be prepared as standards injected for each unique material.
- An injection of standard should occur at least once for every 10 sample injections.
- Spike is 1 ppm BHT in water and in 1% polysorbate-80 extraction solvent.
- Control sample injections should be run to subtract matrix-associated peaks from consideration.
- Report levels of peaks from samples that are also observed in controls ≥ 50% higher than in controls.
- Mass spectrometric detection scan range is 30 to 600 m/z.
- In cases where quantitation is not possible, semiquantitative values may be reported by reference to responses of suitable standards.
- For semiquantitative analysis, results for peaks with a signal-to-noise ratio > 10 or peaks above area of lowest standard injection should be reported.
Liquid-Liquid Extraction Procedure for Direct Injection
- Use dichloromethane (DCM) as an extraction solvent and phenanthrene-d10 as an internal standard.
- Adjust pH as needed.
- Extract aqueous samples in 1:1 (v/v) ratio with DCM, including internal standard; repeat extraction three times on each aqueous sample aliquot.
- Combine DCM fractions and evaporate to approximately 1 mL; repeat preparation if sample reaches significantly less than 1 mL.
- Reconstitute concentrated extract for analysis with DCM to final volume equal to original sample aliquot volume.
3. Detection of Extracts by GC-MS: Headspace Sampling GC with Mass Spectrometry
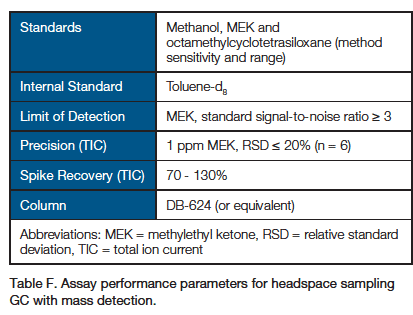
- Other chromatographic instrumentation and conditions may be used to meet assay performance parameters.
- Chromatographic data should be presented using the Total Ion Current (TIC).
- Limit of quantitation (LOQ) should be reported.
- Standards listed in the table are to demonstrate method sensitivity and chromatographic range. Additional known extractable compounds should be prepared as standards injected for each unique material.
- An injection of standard should occur at least once for every 10 sample injections.
- Spike is 1 ppm MEK in water and in 1% polysorbate-80 extraction solvent.
- Control sample injections should be run to subtract matrix-associated peaks from consideration.
- Report levels of peaks from samples that are also observed in controls ≥ 50% higher than in controls.
- Mass spectrometric detection scan range is 30 to 400 m/z.
- In cases where quantitation is not possible, semiquantitative values may be reported by reference to responses of suitable standards.
- For semiquantitative analysis, results for peaks with a signal-to-noise ratio > 10 or peaks above area of lowest standard injection should be reported.
4. Detection of Extracts by Inductively Coupled Plasma with Mass Spectrometric Detection (ICP-MS)
- Instrument and analysis conditions should be optimized to achieve required sensitivity.
- Screen elements identified in ICH Q3D and USP <232>; where applicable, include silicon, tungsten and any additional elements known/suspected to be present in study material.
- The target level of Limit of Detection (LOD) is 20 ppb. The LOD may be lower or higher than 20 ppb depending on the element being detected, the sample matrix, and instrument parameters used. When the LOD is higher than 20 ppb, a justification should be provided.
- Report the LOD obtained for each element detected.
- Limit of quantitation (LOQ) should be reported.
- Standard solutions containing detected elements should be used for recovery study; the recovery should be from 80 to 120%.
- Quantify the detected elements based on calibration curves.
- For the elements that have concentrations higher than DL, report the concentrations and µg/cm2.
- For the elements that are below DL, report the DL and indicate ND (not detected).
- Control sample injections should be run to subtract matrix associated elements from consideration.
Acknowledgements
The authors sincerely thank the more than 40 colleagues from 18 companies in the BPOG Extractables Work Group for their contribution in developing our proposal, and in particular the following individuals: Tony White, Director BioPhorum Operations Group; Gerry McAuley, Facilitator BioPhorum Operations Group; Bobbijo V. Redler, Ph.D. Associate Principal Scientist Merck & Co., Inc.; Nancy Sweeney, Senior Scientist MTS Gallus Biopharmaceuticals; Ping Wang, Ph.D. Principal Scientist Johnson & Johnson; Russell Wong, PhD Sr. Manager Manufacturing Sciences – Raw Materials, Bayer Healthcare; Sally A. Kline, Scientific Director Amgen; Cara Weitzsacker, PhD QC Senior Specialist Bayer HealthCare; Amy J. Stitt, M.S. Scientist I Bristol-Myers Squibb; and Robert Repetto, Senior Director External Affairs Pfizer. The authors appreciate the detailed constructive review of the manuscript from Dr. Duncan Low from Amgen and Dr. Dennis Jenke from Baxter. We also thank Terry Hudson from Genentech, Dr. Michael T. Jones from Pfizer for reviewing the article, and Jim Sayer from Amgen for providing useful input on gamma irradiation.