The impact of paperless validation on the biotechnology industry is massive, and the overall effect is growing as adoption increases. Paperless validation is applicable to every type of validation in biotech: cleaning, process, equipment, facilities, utilities, and many others. Twenty percent of a project’s budget is devoted to validation activities, so streamlining profoundly impacts the...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
The advantages that come with a fully digitalized plant for manufacturing biopharmaceuticals are well documented and showcased throughout the biopharmaceutical industry and will be a featured topic during the 2023...
We are The Culture Club, a self-formed cross-CoP group of individuals who came together to discuss challenges encountered as we transition quality and validation into the digital world and identify ways to influence cultural changes needed within our industry to better enable innovation. As we are not all from the same CoP, we first needed to establish a way to connect and collaborate with all...
On 17 December 2022, more than 100 people from all over the world gathered online for the 2022 China Pharmaceutical Supply Chain Summit hosted by Shanghai ISPE Pharmaceutical Information Company (SPIC). With multiple geopolitical and pandemic related challenges in recent years, having a robust supply chain has increasingly become a strategic advantage for the pharmaceutical industry. The...
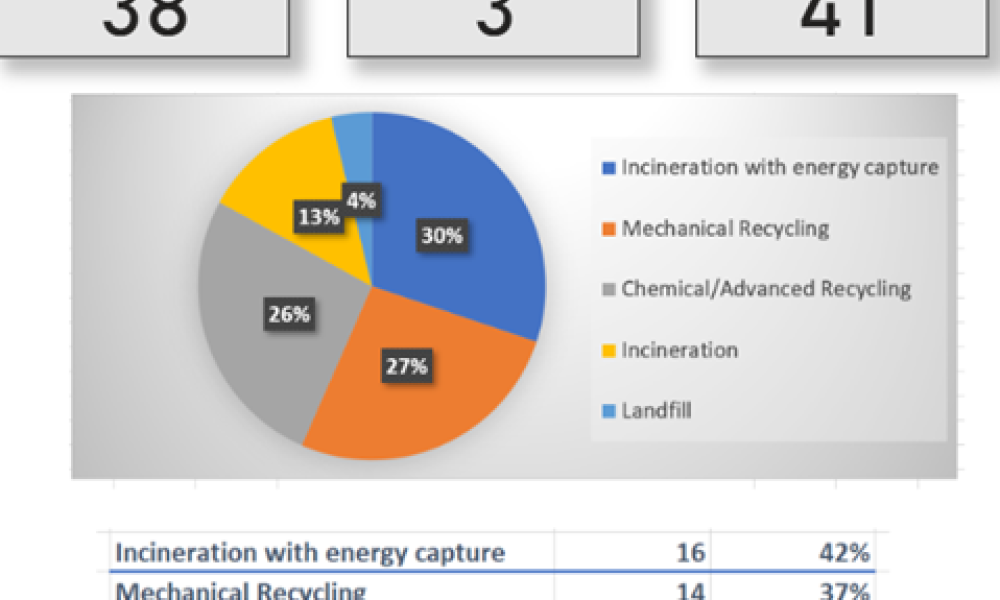
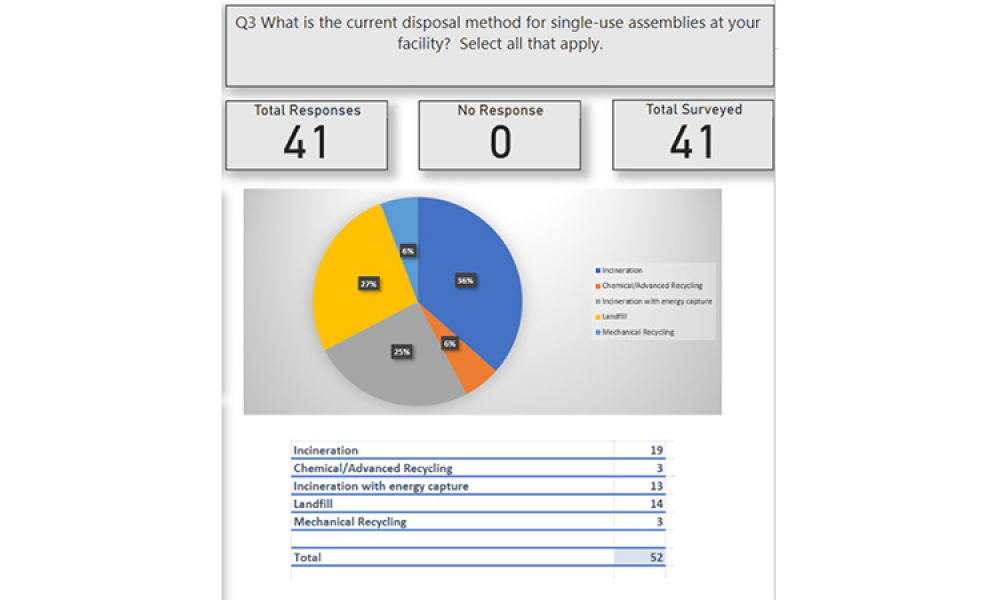
In the iSpeak Blog posting on 14 March 2023, the first part of this blog series introduced the results of a survey that was conducted of users of single-use assemblies. The focus...
Members of the paperless validation subcommittee created this blog post to discuss and recommend “true copy verification practices” for the use of Paperless validation systems to satisfy the current guidance for data integrity, and to discuss how to fully eliminate paper from various validation processes without impacting compliance to these current regulations.
The Biopharmaceutical industry continues to grow and deliver life-changing medicines to patients as evidenced by the number of drug approvals by the FDA year after year. In 2022 alone CDER approved 37 novel drugs, either as new molecular entities (NMEs) under New Drug Applications (NDAs) or as new therapeutic biological products under Biologics License Applications (BLAs). This has all been...
The 2022 ISPE Pharma 4.0™ Emerging Leader Hackathon event was organized by the ISPE Emerging Leaders DACH Affiliate in collaboration with the ISPE Pharma 4.0™ Community of Practice (CoP) Plug and Produce Subcommittee. The goal was to bring together students and young professionals from different fields of study and countries together to explore the emerging challenges and opportunities in...
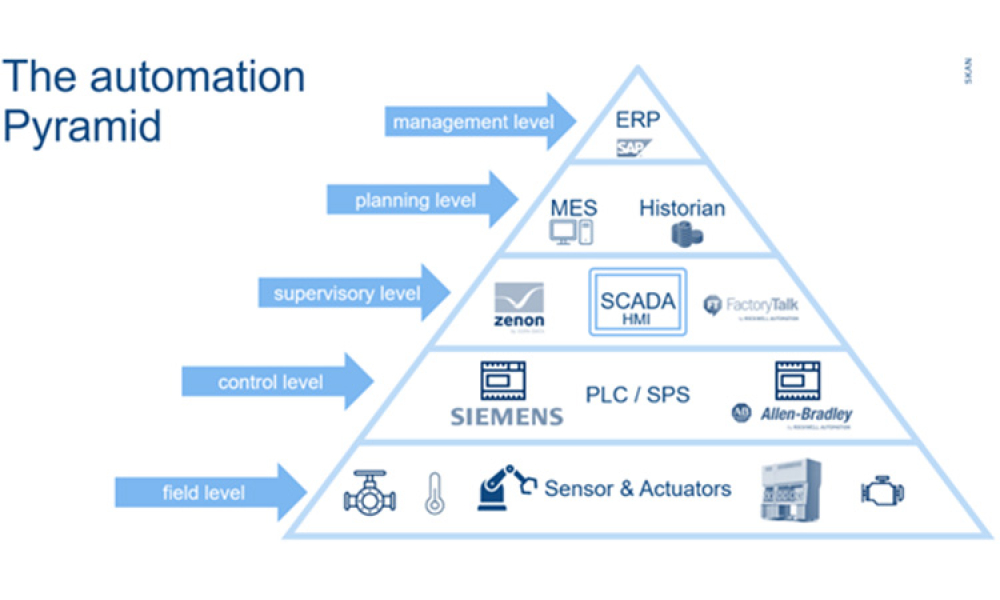
The lifecycle of interfaces is of crucial importance for the interoperability of systems from different technical and organizational areas within or between companies.
Interfaces are the integration points of connected systems for the transmission of master or transaction data including status updates. Interfaces are typically parts of data streams or data hierarchies, e.g., Lab...
The pharmaceutical industry is rapidly evolving. The major trends in the recent years are (1) fully automated production such as continuous manufacturing and (2) the adoption of digitalized processes for paperless production. Both approaches have significant implications for Annex 1 compliance and the role of Contract Manufacturing Organizations (CMOs) in production facilitating these...
Users Perspective on End of Life Management for Single-Use Products used in Bioproduction
ISPE invites Emerging Leaders and Students from all over the globe to join us at this unique virtual event to explore the ISPE Communities of Practice (CoPs) and meet with the leaders of the CoP Steering Committees. This provides an opportunity to engage with industry leaders, learn about the various CoPs, and find out how you as an Emerging Leader or Student can get more involved.
Arriving in the United States at the age of 17 to pursue my dreams was one of the greatest challenges of my life. It was through this experience that I learned the importance of challenging my perspective. This was made possible through my involvement with ISPE, and four years later, I’m proud to announce the launch of Mentor ISPE.
Pharmaceutical and biotech portfolios continue to transition from high volume blockbusters to a diversified set of lower volume, targeted therapeutics. At the same time, many companies are pursuing localized manufacturing to adjust to supply chain challenges and to improve access to medicine. The industry must also address increased pricing pressure due to inflation and government policies....
GAMP® 5 (Second Edition) was published on 29th July 2022 and was presented and discussed at the 2023 ISPE Annual Meeting and at several local...
Here’s How Our Members Intend on Making It a Reality…
Each International Women’s Day, the world comes together to celebrate the social, economic, cultural, and political achievements of women, calling for accelerated equality across borders. International Women’s Day has taken place for over a century, with the first taking place in 1911 and having the support of over a million...
Just off the heels of ISPE’s Facilities of the Future Conference, which took place this past February, ISPE’s Women in Pharma continues to leverage its momentum to amplify the group’s mission to create a more equitable pharmaceutical industry on a grand scale.
The 2023 Facilities of the Future Conference Women in Pharma panel welcomed a full room. Seen from left to right: Carolina Serrano (moderator), Kerren Bergman, Vivianne Arencibia and Jessica Ballinger