Consequently, ISPE recommended that the agency issue a final guidance for a limited, carefully structured pilot program. The program and associated guidance, designed in concert with industry representatives, could clarify requirements and a relative value to the burden. ISPE also recommended that the pilot be based on common mechanisms of engagement. A small but diverse group from industry, for example, could work with the FDA Quality Metrics Team to establish a structured, multiphase approach with measurable goals, milestones, and evaluation points.
As an alternative, ISPE also proposed that the FDA further review the stated goals of the quality metrics program and consider alternative approaches to its guidance, which is currently based on industry submission of harmonized data elements. ISPE indicated its willingness to work with the agency to develop such an alternative approach.
ISPE representatives had an additional series of interactions with the FDA, during which the agency requested further explanation of ISPE’s recommendations for definitions related to the 2016 draft guidance for lot acceptance, product quality complaint, and invalidated out of specification (OOS) rates. ISPE also shared some preliminary thinking about the limited, carefully structured pilot.
To develop ISPE’s thinking further, ISPE conducted a workshop that included representatives from several companies that participated in its Quality Metrics Pilot Program, Waves 1 and 2,
,
in October 2017. Using learnings from these interactions and workshop feedback, ISPE submitted further recommendations
to the FDA docket, including:
- Further elaboration of ISPE recommendations for definitions of lot acceptance, product quality complaint, and invalidated OOS rates
- Alternative programs meeting FDA and industry goals
- Carefully structured FDA pilot program
Advancing Pharamceutical Quality
At the participants’ workshop, ISPE drafted preliminary thoughts for processes to achieve FDA goals. Suggested design elements included:
- Voluntary
- Phased
- Well-defined assessment criteria
- Inclusion of incentives/recognition
ISPE further recommended that the voluntary program could be self-propagating through engagement of early adopters/change ambassadors to show industry leadership and commitment. To encourage participation, benefits should be demonstrable and could include those recognized to support a continual improvement culture, such as reduced inspections and optimized post-approval filing processes.
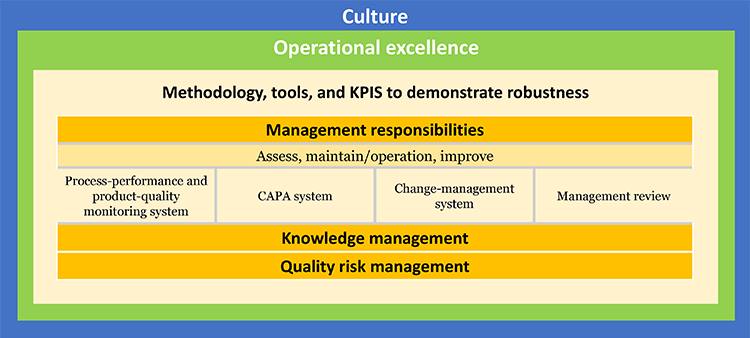
Instead of submitting standardized quality metrics, ISPE also proposed that companies provide information “in advance of or in lieu of an inspection,” as stated in the FDA Safety and Innovation Act,
which could include operational excellence elements. Further discussion on this early thinking was identified as a key next step.
This alternative program, tentatively entitled “Advancing Pharmaceutical Quality,” would demonstrate value to industry, regulators, and patients by integrating tools and experience from quality culture, quality metrics, and operational excellence. It could be based on existing programs such as the US Occupational Safety and Health Administration Voluntary Protection Program and the FDA Case for Quality Program. It could also take elements from the UK Medicines and Healthcare Products Regulatory Agency pre-inspection information-request process. Further exploration and discussion between industry and FDA is needed to advance these ideas.
Proposed deliverables from this program include:
- Assessment and continual improvement tools
- Benchmarking forums
- Interactions with regulators, especially the FDA
- Educational tools (conferences, articles)
- Industry engagement workshops
“Advancing Pharmaceutical Quality” could provide value to industry by encouraging self-improvement as well as supplier improvement, even if it is not adopted by regulators. Parts of the program could be considered for adoption by regulators, either initially or after implementation, leading to benefits to industry through regulatory interaction and potential regulatory relief.
Proposed goals are to:
- Enable and foster industry ownership of quality beyond compliance
- Integrate quality, cultural, and operational excellence
- Support and incentivize continual
- Promote efficient use of resources by improving operational execution
- Increase reliability of product supply (improve could work with the FDA Quality Metrics Team to supply chain robustness
- Fuel benchmarking, sharing, and among companies
The vision for this program would remain that elucidated by the Research Director of the FDA’s Center for Drug Evaluation, Janet Woodcock: “A maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drugs without extensive regulatory oversight.”
Program Design
A project core team—senior representatives from companies with a range of responsibilities, including quality, operations, information technology, and regulatory—and a series of subteams are working on different elements of the program:
- Alternative program design
- Integrated approach to quality and operational excellence
- Applied quality cultural excellence
- Evaluating FDA Quality Metrics submission portal