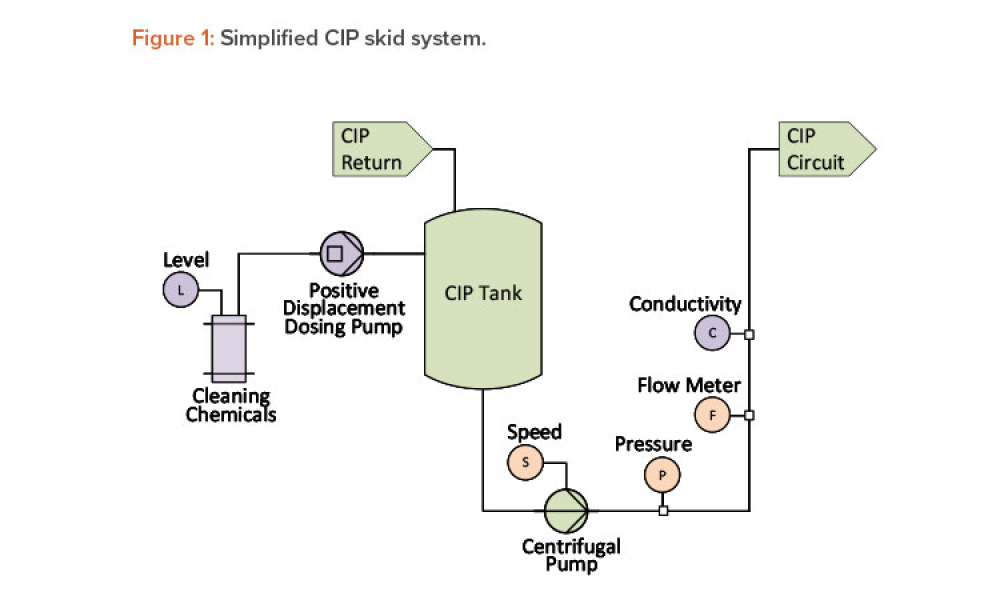
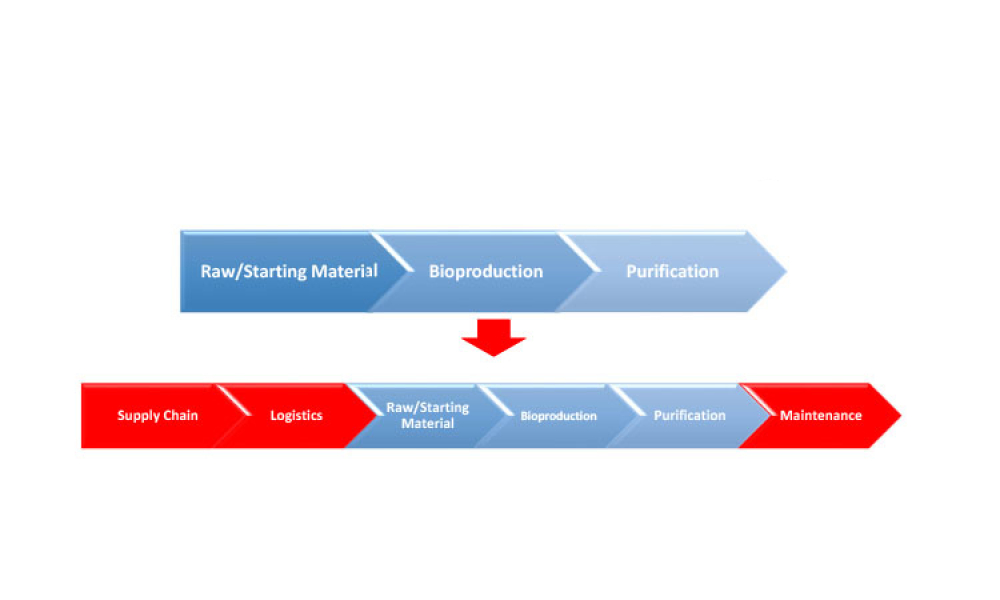
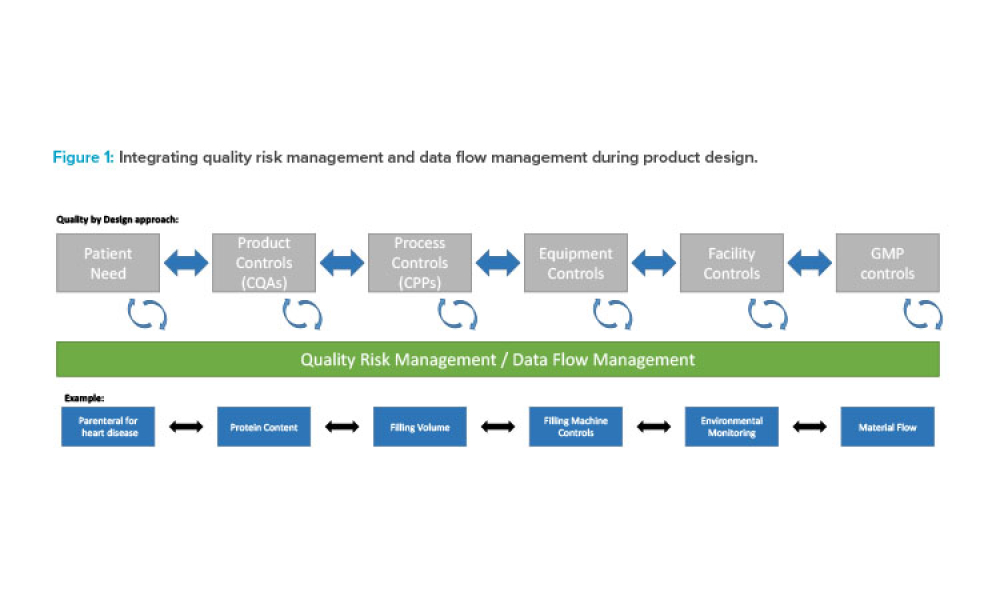
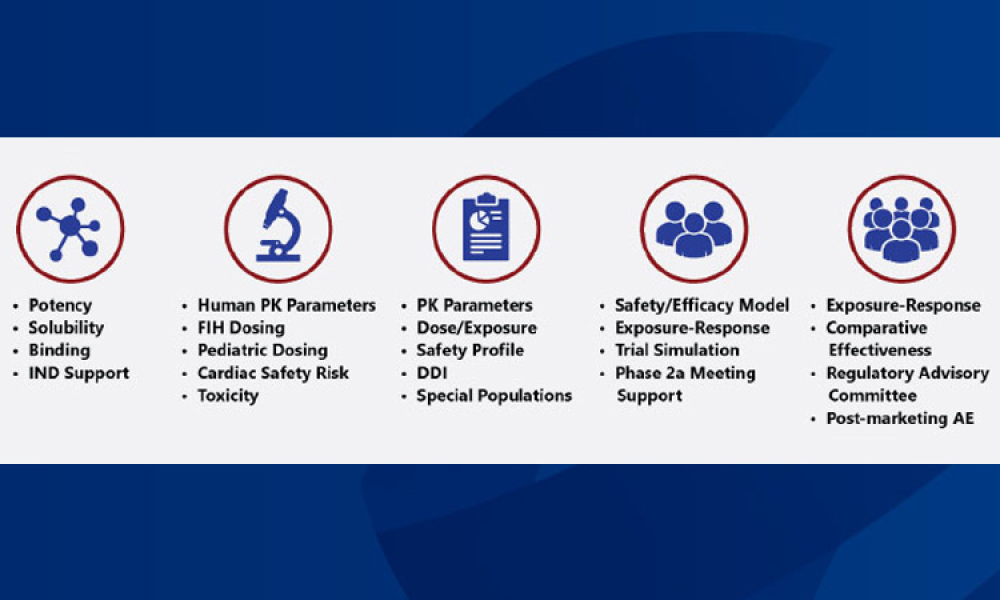
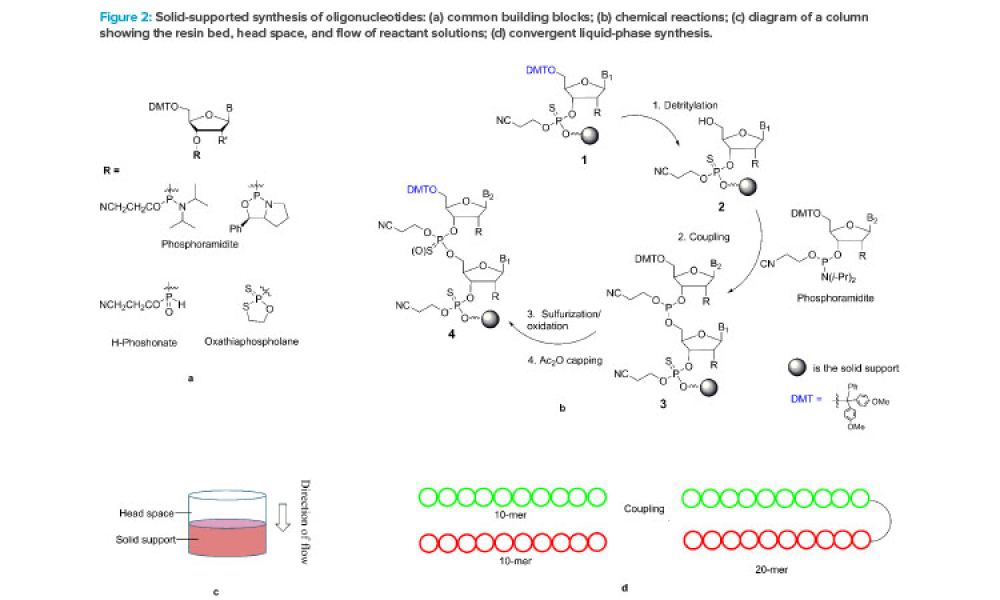
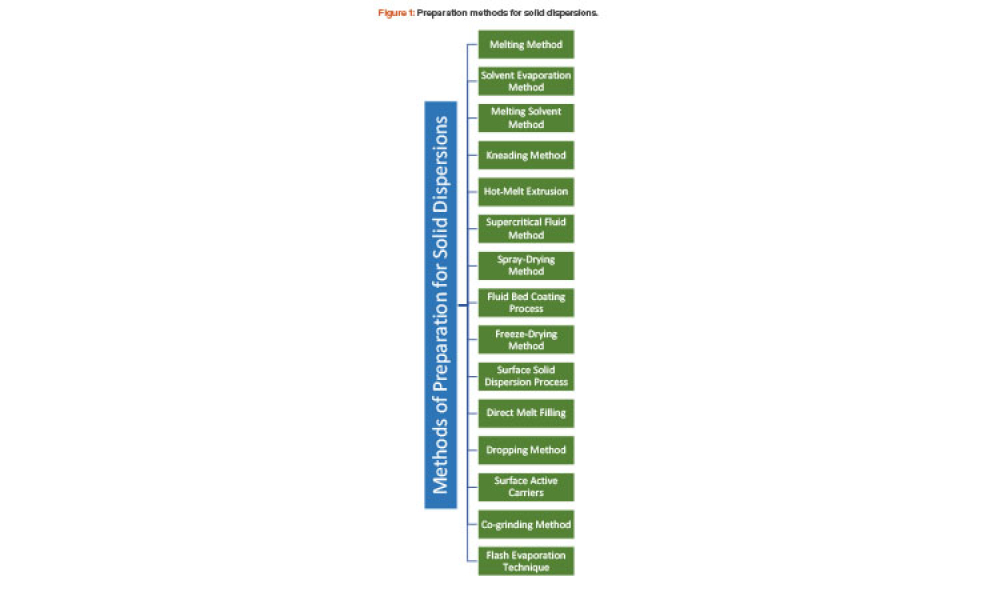
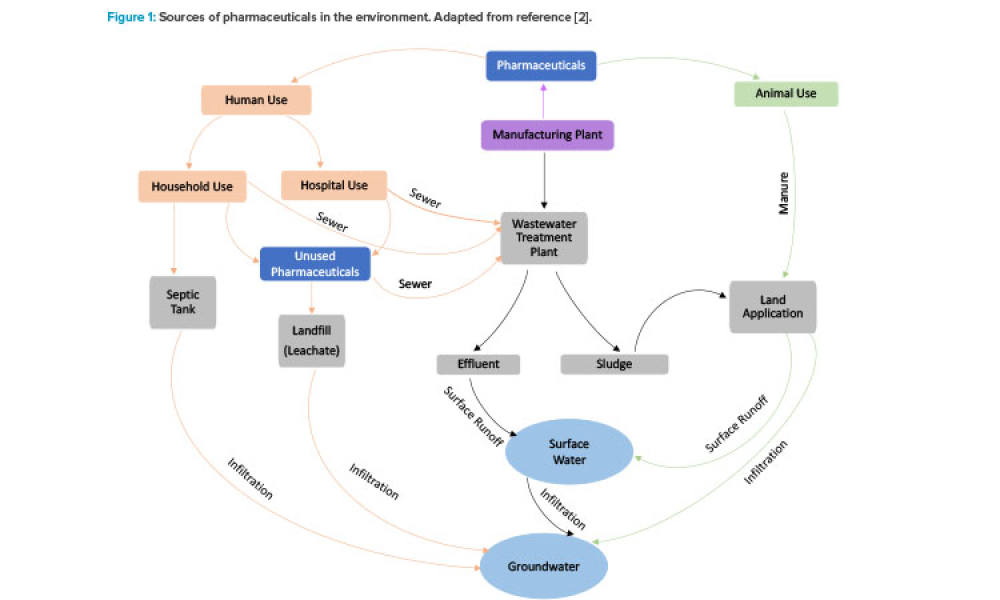
It has long been recognized that one of the greatest sources of contamination in aseptic manufacturing is the transfer of materials into and out of cleanrooms. Regulatory frameworks such as the EU’s Annex 1, FDA’s Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing, ISO 14644-5, and USP <1072> all emphasize the importance of having defined, validated procedures...
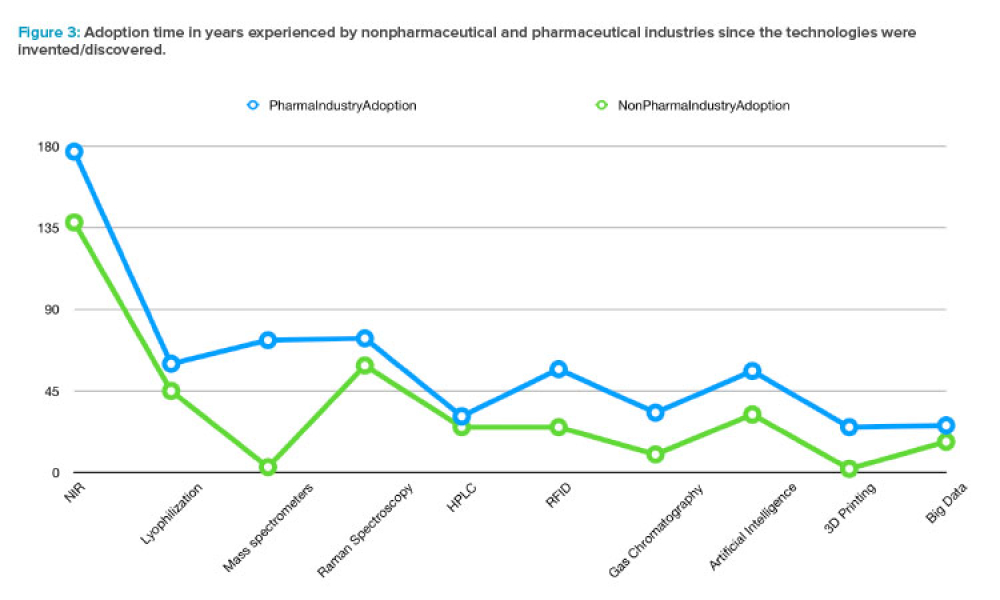
In April 2023, ISPE launched a survey to understand the sources of barriers to technological innovation within the pharmaceutical industry. This survey is part of an expansive and significant initiative by ISPE, Enabling Global Pharma Innovation: Delivering for Patients, which aims to promote consistent and harmonized interpretation and implementation of guidelines issued by the International...