Features
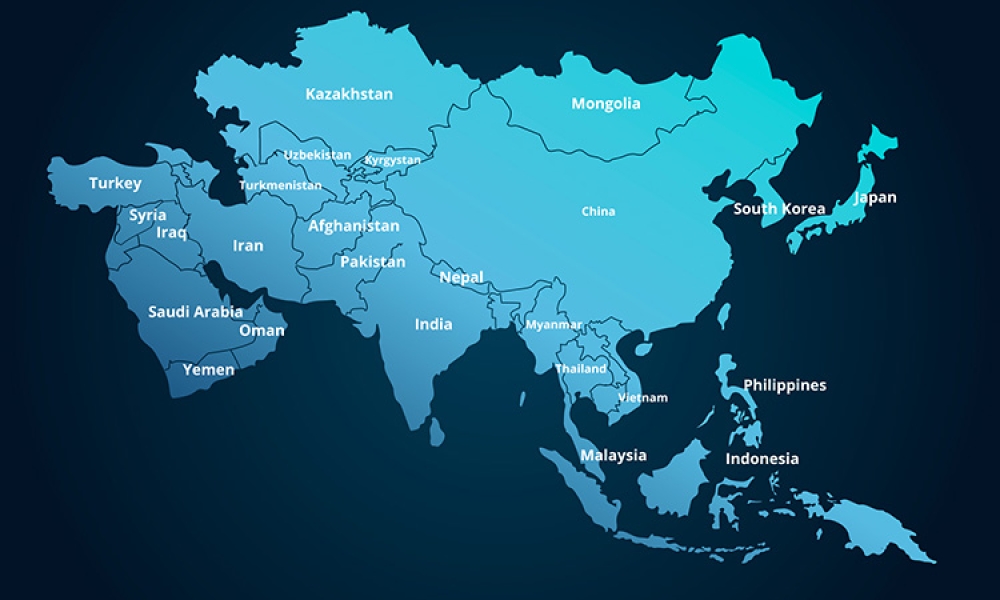
The Asia Pacific region (APAC), like any large territory, encompasses a blend of well-established and early-stage economies, diverse healthcare systems, and differences in language, culture, politics, and technology adoption. APAC’s size and complexity has created new challenges and opportunities for the pharmaceutical industry as nations work together to meet the manufacturing needs for medical products.