This article describes an amazing story of self-sufficiency: how Thailand has managed to develop six different Covid 19 vaccines in a very short time. This story describes how the Thai Government, The Royal Family, the Universities and private companies are working together to benefit the nation, three of these are using new technology developed in Thailand.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are articles from 10 years ago: the year was 2012, and ISPE members were reading about a holistic approach to pharmaceutical manufacturing, defining metrics and intelligence, battling counterfeit medicines, and more.
The very definition of gratitude seems lovely – be thankful, show appreciation and return kindness.
Every year, the world rallies together on March 8th in support of International Women’s Day. This year, the movement is diving into the importance of creating a world free of bias, stereotypes, and discrimination. Below, two members of ISPE’s Women in Pharma Steering Committee share their perspective on biases, and how they navigated these experiences throughout their careers:
Fatima Jacoba Mancilla Islas is a Foundation Board Member of the ISPE Mexico Affiliate and is currently a Validation Manager for Probiomed SA de...
The Women in Pharma Steering Committee Discuss Biases for International Women’s Day
Looking back on my career, I can honestly say, with a genuine smile, that, I am completely satisfied. I want for nothing, and for that I am overwhelmingly grateful and incredibly proud.
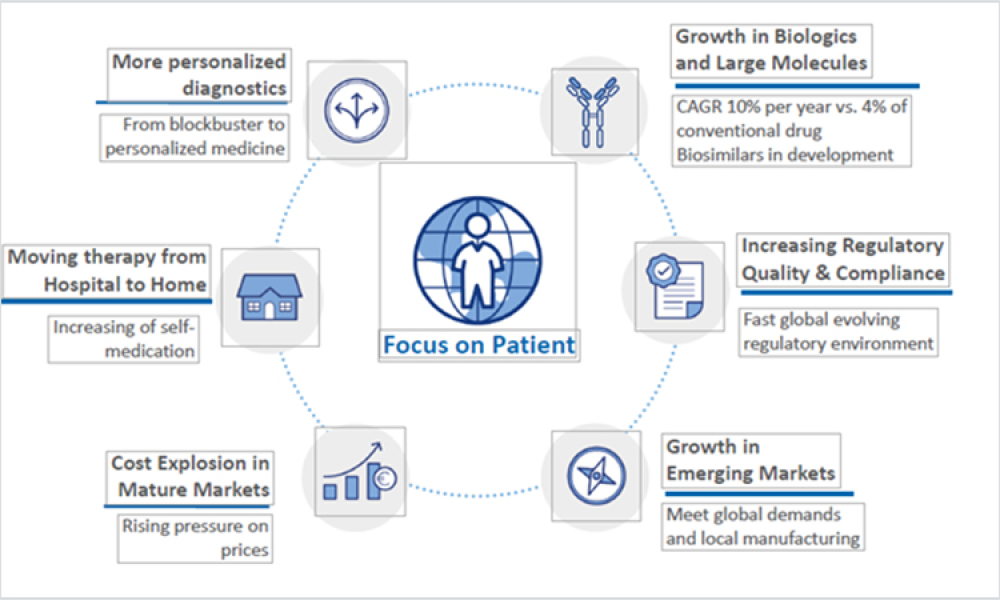
The COVID-19 pandemic has significantly disrupted the biopharma industry megatrends and the world in ways that could not have been predicted. As a result, it forced world leaders, global economists, innovators, and suppliers to collaborate, rethink outdated processes, and innovate together to combat the virus for one common goal, to save human lives. It was by far a pinnacle moment and a great...
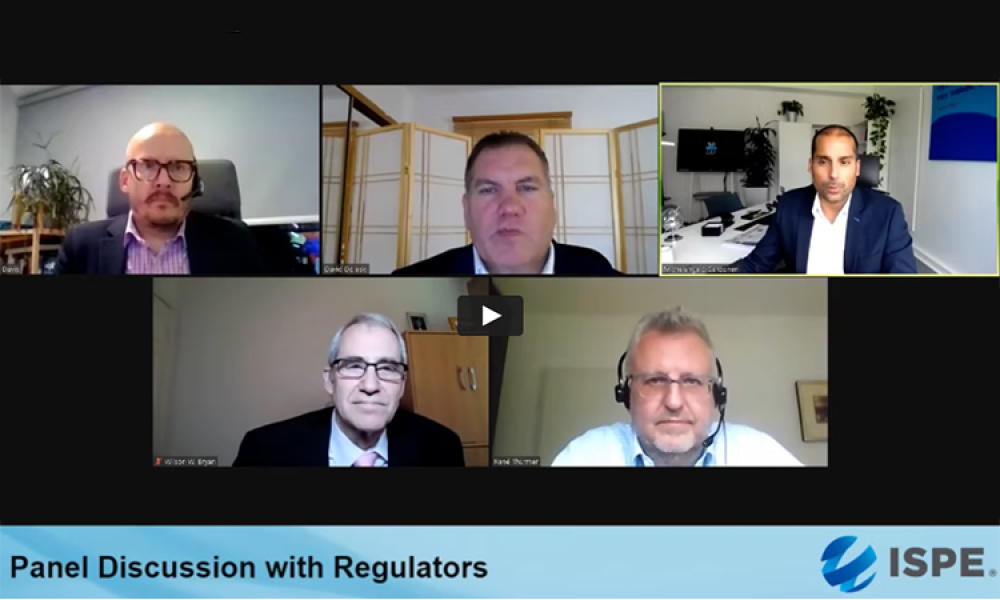
A panel of regulators representing BfArm, Germany; the Therapeutic Goods Administration, Australia; and the US FDA took place at the 2021 ISPE Biotechnology Conference and Workshop. The...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from January 2022. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
In each issue of Pharmaceutical Engineering®, we introduce a member of the ISPE staff who provides ISPE members with key information and services. Meet Lynda Goldbach, Manager of Publications, Publications Department.
Oxana Pryanichnikova is the Vice-Chair of ISPE Eurasian Affiliate with intensive involvement in the foundation of local ISPE organization since 2018. She is the Chief Business and Operating Officer of DXDO with more than 20 years in Software and Hardware Sales, Track&Trace projects, Business Development, planning and executing...
2022 ISPE Facilities of the Future Conference
Executive Forum Panel Discusses Future Pandemic Planning & Other Topics
The Executive Forum Panel at the 2022 ISPE Facilities of...
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are articles about aseptic to help you get ready for the 2022 ISPE Aseptic Conference in March. Here is a selection of some recent articles, including coverage of the popular regulatory panel session at the 2021 Aseptic Conference, the ISPE Barrier Survey, and more.
It was March 2020 and my first year serving on the ISPE Aseptic Conference Planning Committee. I was brimming with excitement of the possibility to introduce speakers and facilitate discussions at this conference I had enjoyed and learned from multiple times as an attendee. Little did I know how much I would get to participate, as my fellow committee members were restricted from traveling to...
The ISPE 2022 Facilities of the Future Conference Opening Plenary provided views of lessons learned during the pandemic that will help the pharmaceutical industry to accelerate development of new...
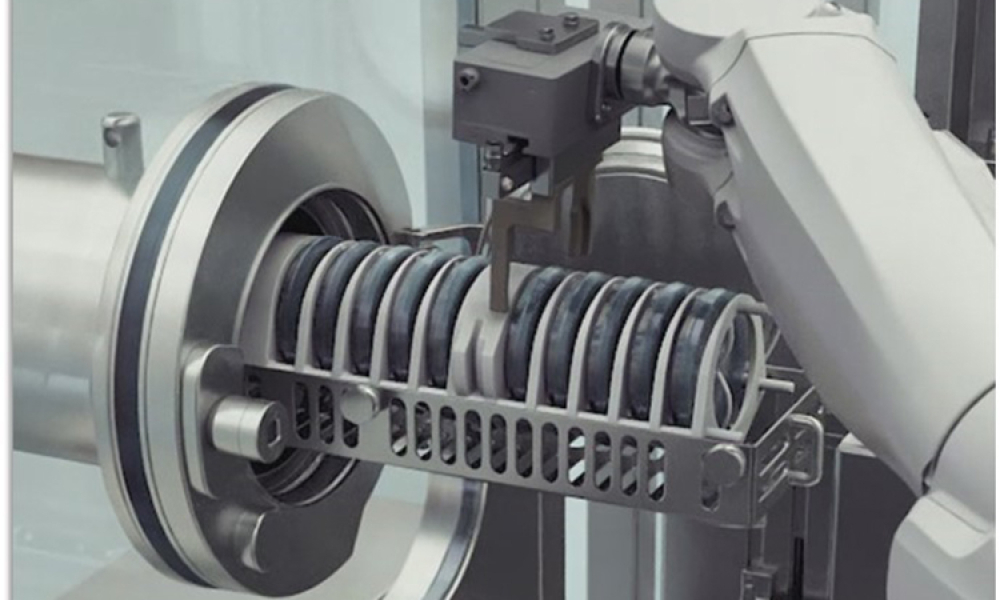
Aseptic filling of parenterals is one of the most challenging tasks within pharmaceutical manufacturing. A wide range of topics falls under this umbrella, from more engineer-driven topics (e.g., filling accuracy and machine performance) to key production questions (such as how to ensure the process remains sterile and compliant within the law and regulatory guidelines). All this is influenced...
The ATMPs - Autologous Cell Therapy Guide focuses primarily on manufacturing facility development and design for autologous cell therapies for parenteral use. This Guide provides an overview of the critical aspects of ATMP facility design as well as the key relationship between current process/facility attribute alignment and how that changes in the ATMP space.
Biotech is adapting and evolving to have more automation, digitalization, and we need to be ready for the new challenges. Join us at the 2022 ISPE Facility of the Future Conference...