The compliance implications of this concept are discussed below.
As a corollary, all of these models can also be applied to the relationship between manufacturer and system vendor. Generally speaking, through an appropriate network security policy, companies could temporarily connect system vendors to machines hosted on the company’s network, thereby allowing direct support from the system experts.
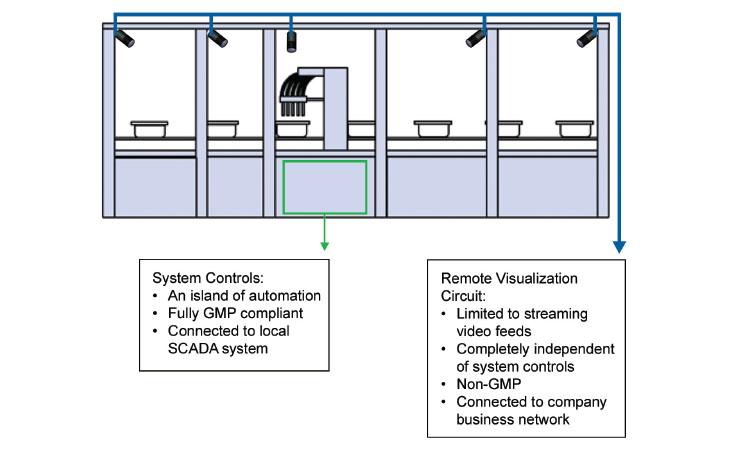
3. Camera Architecture—Separate and Distinct Room Control Logic
The concept of cameras embedded within a manufacturing system is nothing new—industrial cameras have been used as critical components in pharmaceutical lines for decades. Generally, these cameras, especially “smart” cameras, are qualified, validated measurement devices, fully integrated into the machine’s control logic.
In this article we are proposing a wholly separate camera application. The network-enabled streaming cameras operate in complete isolation from the system architecture. Since they do not contribute to product inspection and any pass/fail decision, there is no intrinsic need for such devices to be fully validated and compliant. The proposal is that such cameras would be used only to provide operational information and not for any product quality-related decision (Figures 1 and 2).
ISPE’s
Concept and Discussion Papers reflect the experience and input of their contributors, represent industry best practices, and cover a range of topics. They are available exclusively to ISPE members.
4. Compliant Data Handling and Storage
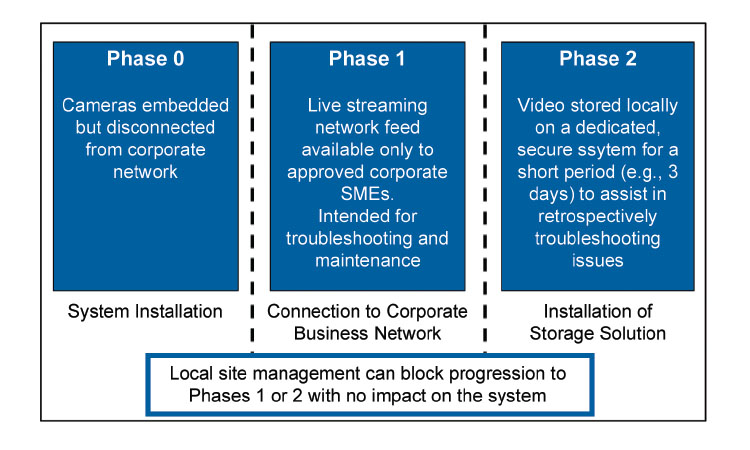
In addition to live streaming to relevant employees across a company’s global network, embedded cameras present another opportunity with respect to data storage considerations. Long-term data storage is for the most part not pragmatic when costs are considered; however short-term data storage, perhaps over a matter of days, is eminently feasible. In a manner very similar to contemporary dashboard camera solutions for road vehicles, video could be stored for a short time in an effort to capture adverse events, particularly equipment malfunctions. This could assist enormously in subsequent root-cause investigations, especially if the video coverage of a given line is suitably comprehensive.
FDA 21 CFR Part 11
compliant video storage solutions are technically feasible; however, if the video is intended only for system troubleshooting and is not used for drug product lot release, we believe such a solution can be implemented without being considered an integral part of the validated production platform (Figures 3 and 4).
5. Challenges in the Pharmaceutical Manufacturing Space
The technologies discussed in this document are readily available and, in some cases have traction in other major industrial markets. The pharmaceutical industry has some unique challenges that need to be met. These may be further compounded by legal considerations associated with the jurisdiction of a particular manufacturing location.
First, pharmaceutical manufacturing generally requires the use of cleanroom manufacturing protocols. Mobile peer-to-peer communication solutions must reside permanently within the clean space. The requirements of the cleanroom place constraints on the design and materials used in the construction of an approved device. The high density of piping and supporting utilities in a typical plant generally diminishes cellular reception, forcing the need for an effective local wireless network.
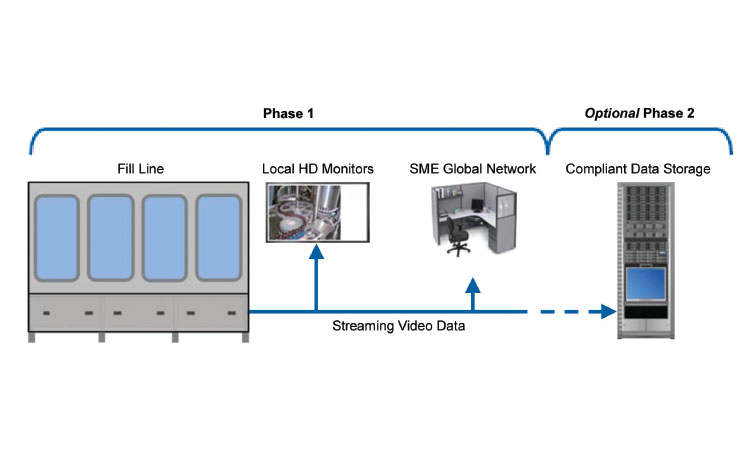
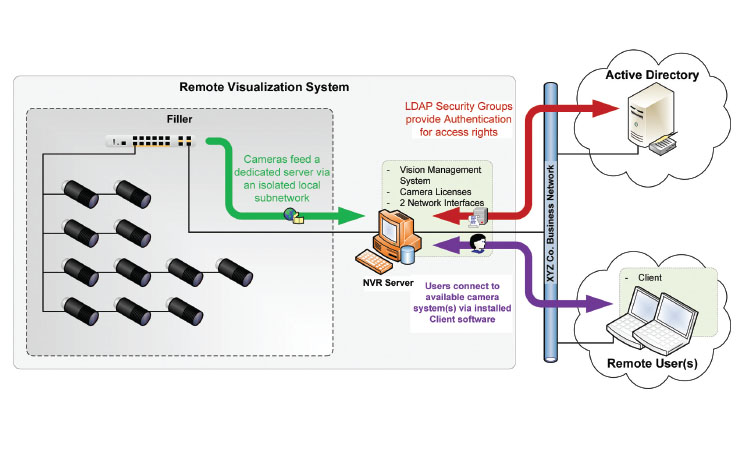
Figure 5: This image shows the overall architecture used at one company. A server, separate and distinct from the manufacturing system, is used to manage all of the camera feeds. Software on the server controls user access and also allows optional, programmable video data storage capability.